主要功能
采用独创的板载芯片 LED 阵列技术,用 6 种不同波段的激发光作为测量光、光化光、饱和脉冲、单周转饱和闪光与多周转饱和闪光
具备比 PAM-2500 高 200 倍的灵敏度
优化设计用于很稀的悬浮液(藻液、叶绿体悬浮液)测量
专用叶夹可用于高等植物/大型海藻等叶片状样品的测量
标准的 PAM 测量功能、复杂的多相荧光上升动力学拟合分析、驰豫动力学分析
特别适合状态转换研究、“非活性PSII”(“Inactive PS II”)研究
超快时间分辨率达到 10 ms,由此利用独特的 O-I1 相(O-J相)拟合分析用于分析PSII反映中心异质性分析,得出 PS II 光合单位的连接性参数(p和J),速率常数(Tau)和两种不同类型 PS II(Type 1 和Type 2)的光学截面积(Sigma(II)λ)等参数
新增 PSII 有效光强 PAR(II)、经过 PSII 的绝对电子传递速率 ETR(II)λ 等全新的光合参数。
专业的操作软件,用于复杂的拟合分析
测量参数
Fo, Fm, F, Fm', Fv/Fm, Y(II), qP, qN, NPQ, Y(NO), Y(NPQ), ETR, ETR(II)λ, p, J, Tau, Sigma(II)λ, PAR、PAR(II) 等
应用领域
主要用于各种藻类的深入光合作用机理研究,用适合的波长、全新的测量、全新的参数进行蓝藻、绿藻、硅藻、甲藻、红藻、隐藻等的深入研究。如选配高等植物附件,也可实现对高等植物叶片的测量。
主要技术参数
测量光:提供 400、440、480、540、590 和 625 nm 的脉冲调制测量光,20 个强度选择,14 个频率选择。
光化光:提供 440、480、540、590、625 nm 和 420-640 nm(白光)连续光化光照,最大光强 4000 μmol m-2 s-1;单周转饱和闪光的最大强度 200 000 μmol m-2 s-1,持续时间 5-50 μs可调;多周转饱和闪光强度 10 000 μmol m-2 s-1,1-800 ms可调。
远红光:725 nm。
信号检测:PIN-光电二极管,带特制锁相放大器(专利设计),最大时间分辨率 10 μs。
Multi-Color-PAM的功能介绍
光系统 II 的相对电子传递速率 rETR 是很常用的一个参数。rETR = PAR × Y(II) × ETR-factor,其中 ETR-factor 是指光系统II吸收的光能占总入射 PAR 的比例。在绝大多数已发表的文献中,均没有试图去测定 ETR-factor,只是简单地假定跟 “模式叶片” 相同,即有 50% 的 PAR 分配到光系统 II,84% 的 PAR 被光合色素吸收。因此在已有的文献中,rETR一般是用公式 rETR = PAR × Y(II) × 0.84 × 0.5 来计算的。
近期,利用多激发波长调制叶绿素荧光仪 MULTI-COLOR-PAM 可以实现光系统II的绝对电子传递速率 ETR(II)λ 的测量。首先需要利用 MULTI-COLOR-PAM 测定某个波长下的光系统II功能性光学截面积 Sigma(II)λ(单位nm2)(其中λ为波长),然后求出光系统II的量子吸收速率 PAR(II) = Sigma(II)λ × L × PAR = 0.6022 × Sigma(II)λ× PAR。其中 L 为阿伏伽德罗常数,系数 0.6022 是将 1 μmol quanta m-2 (即 6.022 × 1017 quanta m-2)转换为 0.6022 quanta nm-2,PAR(II) 的单位为 quanta/(PSII × s)。接下来就可以计算 ETR(II)λ = PAR(II) × Y(II)/Y(II)max,其中 Y(II)max 是经过暗适应达到稳态后的光系统II的量子产量,也就是 Fv/Fm×ETR(II) 的单位为 electrons/(PSII × s)。
传统的调制叶绿素荧光仪一般只能提供一种或两种颜色的光源,如发出白光的卤素灯、发出蓝光的蓝色 LED 或发出红光的红色 LED 等。用不同颜色的光测量的结果可能会有不同,如图 1A 所示,用蓝光(440 nm)和红光(625 nm)测量绿藻小球藻的快速光曲线有非常显著的差别,蓝光照射下的 rETRmax 显著小于红光照射下,且在较强的光曲线 rETR 有轻微下降趋势,这说明蓝光的更容易引发光抑制 (Schreiber, Klughammer et al. 2011, Schreiber, Klughammer et al. 2012)。由此可以推测,过去文献报道的很过实验结果,可能会存在由于采用的激发光源不同而引起的错误理解。
如上文所述,利用 MULTI-COLOR-PAM,已经可以测量绝对电子传递速率 ETR(II)λ。如果用 ETR(II)λ 来绘制快速光曲线会出现什么结果呢?图 1B 是将图 1A 的结果转换成绝对电子传递速率后得到的结果,可以看出无论是照射蓝光还是照射红光,其绝对电子传递速率是一致的。由此证明图 1A 中结果的差异是由于不同波长下藻细胞的光系统 II 功能性光学截面积 Sigma(II)λ 的大小不同引起的 (Schreiber, Klughammer et al. 2011, Schreiber, Klughammer et al. 2012)。这种利用绝对电子传递速率 ETR(II)λ 绘制的快速光曲线在未来的科研中可能会发挥越来越重要的作用。
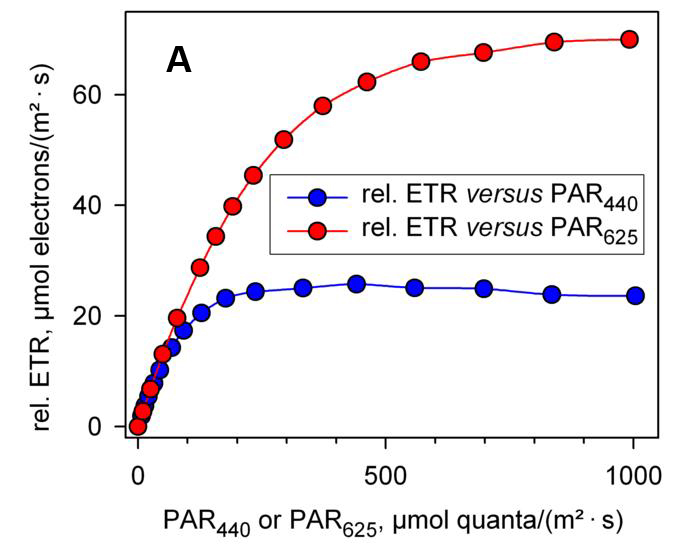 | 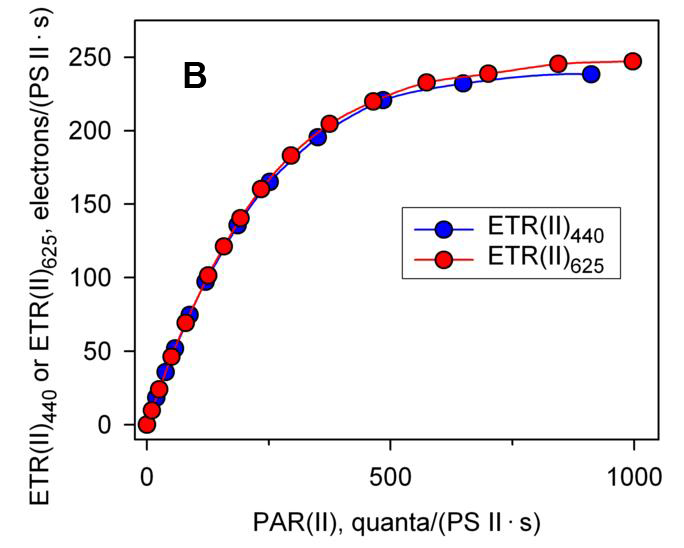 |
| 图1 利用相对电子传递速率(A)和绝对电子传递速率(B)分别绘制的快速光曲线(引自Schreiber et al., 2012) | |
| 利用 MULTI-COLOR-PAM 分别以蓝光(440 nm)和红光(625 nm)作为光化光源,测量小球藻(Chlorella sp.)的快速光曲线。 | |
| 图A中,rETR 的计算采用 0.42 作为 ETR factor。 | |
| 图B中,蓝光和红光激发下获得的光系统II功能性光学截面积 Sigma(II)λ 分别为 4.547 和 1.669 nm2,计算绝对电子传递速率 ETR(II)440 和 ETR(II)625 的 Fv/Fm 分别为 0.68 和 0.66。 | |
选购指南
一、悬浮样品测量基本款
系统组成:通用型主机,标准版检测单元,悬浮液的光学单元,数据线,工作台,软件等
 |
| 悬浮样品测量基本款 |
二 、高等植物叶片测量基本款
系统组成:通用型主机,标准版检测单元,特制叶片夹,数据线,工作台,软件等
 |
| 高等植物叶片测量特制叶夹 |
三、其他可选附件
1,ED-101US/T: 控温装置,安装在 ED-101US/MD 上,为悬浮液控温;可外接循环水浴来控温,
2,US-SQS/WB: 球状微型光量子探头,可插入样品杯中测量 PAR;由主机 DUAL-C 控制。
3,PHYTO-MS:磁力搅拌器,连接到光学单元 ED-101US/MD 的底部对悬浮液进行搅拌。
产地:德国WALZ
参考文献
数据来源:光合作用文献 Endnote 数据库,更新至 2021年 1 月,文献数量超过 10000 篇
原始数据来源:Google Scholar
Schreiber, U. and C. Klughammer (2021). "Evidence for variable chlorophyll fluorescence of photosystem I in vivo." Photosynthesis Research.
Schansker, G. (2021). "Kinetic characterization of the interaction of NO with the S2 and S3 states of the oxygen-evolving complex of Photosystem II." bioRxiv: 2021.2001.2010.426130.
Yun, J., et al. (2020). "Transcriptomic analysis of Chlorella sp. HS2 suggests the overflow of acetyl‐CoA and NADPH cofactor induces high lipid accumulation and halotolerance." Food and Energy Security.
Andrzejczak, O. A., et al. (2020). "The Hypoxic Proteome and Metabolome of Barley (Hordeum vulgare L.) with and without Phytoglobin Priming. ." Int. J. Mol. Sci(21): 1546.
Chen, Y., et al. (2020). "Astaxanthin biosynthesis in transgenic Dunaliella salina (Chlorophyceae) enhanced tolerance to high irradiation stress." South African Journal of Botany 133: 132-138.
Pavaux, A.-S. (2020). "Chemical Ecology of the toxic dinoflagellate Ostreopsis cf. ovata in N.W. Mediterranean Sea." 学位论文.
Smolova, T., et al. (2020). "Cortical photosynthesis as a physiological marker for grape breeding: methods and approaches." BIO Web of Conferences 25: 02018.
Terentyev, V. V., et al. (2020). "The Main Structural and Functional Characteristics of Photosystem-II-Enriched Membranes Isolated from Wild Type and cia3 Mutant Chlamydomonas reinhardtii." Life(10): 63.
Zhang, X., et al. (2020). "Photosynthetic Properties of Miscanthus condensatus at Volcanically Devastated Sites on Miyake-jima Island." Plants(9): 1212.
Grund, M., et al. (2019). "Electron balancing under different sink conditions reveals positive effects on photon efficiency and metabolic activity of Synechocystis sp. PCC 6803." Biotechnology for Biofuels 12(1): 43.
Røkke, G. B., et al. (2019). "Unique photosynthetic electron transport tuning and excitation distribution in heterokont algae." PLoS ONE 14(1): e0209920.
Schreiber, U., et al. (2019). "Rapidly reversible chlorophyll fluorescence quenching induced by pulses of supersaturating light in vivo." Photosynthesis Research: 1-16.
Wang, W. and Y. Sheng (2019). "Pseudomonas sp. strain WJ04 enhances current generation of Synechocystis sp. PCC6803 in photomicrobial fuel cells." Algal Research 40: 101490.
Yanykin, D., et al. (2019). "Hydroxyectoine protects Mn-depleted photosystem II against photoinhibition acting as a source of electrons." Photosynthesis Research: 1-15.
Chartrand, K. M., et al. (2018). "Living at the margins–The response of deep-water seagrasses to light and temperature renders them susceptible to acute impacts." Marine environmental research.
Goessling, J. W., et al. (2018). "Modulation of the light field related to valve optical properties of raphid diatoms: implications for niche differentiation in the microphytobenthos." MARINE ECOLOGY PROGRESS SERIES 588: 29-42.
Khorobrykh, A., et al. (2018). "Photooxidation and photoreduction of exogenous cytochrome c by photosystem II preparations after various modifications of the water-oxidizing complex." Photosynthetica: 1-10.
Li, F., et al. (2018). "Diatom performance in a future ocean: interactions between nitrogen limitation, temperature, and CO2-induced seawater acidification." ICES Journal of Marine Science.
Lin, L., et al. (2018). "Electrochemical oxidation of Microcystis aeruginosa using a Ti/RuO2 anode: contributions of electrochemically generated chlorines and hydrogen peroxide." Environmental Science Pollution Research.
Miao, H., et al. (2018). "Calcification Moderates the Increased Susceptibility to UV Radiation of the Coccolithophorid Gephryocapsa oceanica Grown under Elevated CO 2 Concentration: Evidence Based on Calcified and Non‐Calcified Cells." Photochemistry and Photobiology.
Morelle, J. and P. Claquin (2018). "Electron requirements for carbon incorporation along a diel light cycle in three marine diatom species." Photosynthesis Research 137(2): 201-214.
Morelle, J., et al. (2018). "Annual Phytoplankton Primary Production Estimation in a Temperate Estuary by Coupling PAM and Carbon Incorporation Methods." Estuaries and Coasts: 1-19.
Nikkanen, L., et al. (2018). "Regulation of chloroplast NADH dehydrogenase-like complex by NADPH-dependent thioredoxin system." bioRxiv: 261560.
Preuss, M. and G. C. Zuccarello (2018). "Comparative studies of photosynthetic capacity in three pigmented red algal parasites: Chlorophyll a concentrations and PAM fluorometry measurements." Phycological Research 0(0).
Sung, M.-G., et al. (2018). "Wavelength shift strategy to enhance lipid productivity of Nannochloropsis gaditana." Biotechnology for Biofuels 11(1): 70.
Ternon, E., et al. (2018). "Allelopathic interactions between the benthic toxic dinoflagellate Ostreopsis cf. ovata and a co-occurring diatom." Harmful Algae 75: 35-44.
Zavřel, T., et al. (2018). "Effect of carbon limitation on photosynthetic electron transport in Nannochloropsis oculata." Journal of Photochemistry and Photobiology B: Biology 181: 31-43
Béchet, Q., et al. (2017). "Modeling the impact of high temperatures on microalgal viability and photosynthetic activity." Biotechnology for Biofuels 10(1): 136.
Havurinne, V. and E. Tyystjärvi (2017). "Action spectrum of photoinhibition in the diatom Phaeodactylum tricornutum." Plant and Cell Physiology: pcx156.
Kalaji, M. H., et al. (2017). Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications, CRC Press.
Lamb, J. J. and M. F. Hohmann-Marriott (2017). "Manganese acquisition is facilitated by PilA in the cyanobacterium Synechocystis sp. PCC 6803." PLoS ONE 12(10): e0184685.
Røkke, G., et al. (2017). "The plastoquinone pool of Nannochloropsis oceanica is not completely reduced during bright light pulses." PLoS ONE 12(4): e0175184.
Savchenko, T., et al. (2017). "The hydroperoxide lyase branch of the oxylipin pathway protects against photoinhibition of photosynthesis." Planta: 1-14.
Shin, W.-S., et al. (2017). "Complementation of a mutation in CpSRP43 causing partial truncation of light-harvesting chlorophyll antenna in Chlorella vulgaris." Scientific Reports 7(1): 17929.
Laviale, M., et al. (2016). "The importance of being fast: comparative kinetics of vertical migration and non-photochemical quenching of benthic diatoms under light stress." Marine Biology 163(1): 1-12.
Murphy, T. E., et al. (2016). "A radiative transfer modeling approach for accurate interpretation of PAM fluorometry experiments in suspended algal cultures." Biotechnology Progress: n/a-n/a.
Shin, W.-S., et al. (2016). "Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity." Journal of Applied Phycology: 1-10.
Yanykin, D. V., et al. (2016). "Trehalose protects Mn-depleted photosystem 2 preparations against the donor-side photoinhibition." Journal of Photochemistry and Photobiology B: Biology 164: 236-243.
He, J., et al. (2015). "Photoinactivation of Photosystem II in wild-type and chlorophyll b-less barley leaves: which mechanism dominates depends on experimental circumstances." Photosynthesis Research: 1-9.
Lin, L., et al. (2015). "Effects of electrolysis by low-amperage electric current on the chlorophyll fluorescence characteristics of Microcystis aeruginosa." Environmental Science and Pollution Research: 1-8.
Polishchuk, A., et al. (2015). "Cultivation of Nannochloropsis for eicosapentaenoic acid production in wastewaters of pulp and paper industry." Bioresource Technology 193: 469-476.
Tamburic, B., et al. (2015). "Gas Transfer Controls Carbon Limitation During Biomass Production by Marine Microalgae." ChemSusChem.
Yanykin, D., et al. (2015). "Trehalose stimulation of photoinduced electron transfer and oxygen photoconsumption in Mn-depleted photosystem 2 membrane fragments." Journal of Photochemistry and Photobiology B: Biology 152: 279-285.
Klughammer, C. and U. Schreiber (2014). Apparent PS II absorption cross-section and estimation of mean PAR in optically thin and dense suspensions of Chlorella. Photosynth Res.
Szabó, M., K. Parker, et al. (2014). Photosynthetic acclimation of Nannochloropsis oculata investigated by multi-wavelength chlorophyll fluorescence analysis. Bioresource Technology 167: 521-529.
Szabó, M., D. Wangpraseurt, et al. (2014). Effective light absorption and absolute electron transport rates in the coral Pocillopora damicornis. Plant Physiology and Biochemistry.
Tamburic, B., M. Szabó, et al. (2014). Action spectra of oxygen production and chlorophyll a fluorescence in the green microalga Nannochloropsis oculata. Bioresource Technology 169: 320-327.
Hakkila, K., T. Antal, et al. (2014). "Oxidative stress and photoinhibition can be separated in the cyanobacterium Synechocystis sp. PCC 6803." Biochim Biophys Acta 1837(2): 217-225.
Reigosa, M., D. Wangpraseurt, et al. (2014). "Spectral Effects on Symbiodinium Photobiology Studied with a Programmable Light Engine." PLoS ONE 9(11): e112809.
Schreiber, U. and C. Klughammer (2013). Wavelength-dependent photodamage to Chlorella investigated with a new type of multi-color PAM chlorophyll fluorometer. Photosynthesis Research 114(3): 165-177.
Bernát G, Schreiber U, Sendtko E, Stadnichuk IN, Rexroth S, Rögner M, Koenig F (2012) Unique Properties vs. Common Themes: The Atypical Cyanobacterium Gloeobacter violaceus PCC 7421 is Capable of State Transitions and Blue-light Induced Fluorescence Quenching. Plant & Cell Physiology: in press.
Schreiber U, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of Multi-Color-PAM chlorophyll fluorometer. Photosynthesis Research: in press.
Schreiber U, Klughammer C, Kolbowski J (2011) High-end chlorophyll fluorescence analysis with the MULTI-COLOR-PAM. I. Various light qualities and their applications. PAM Application Notes, 4: 1-19.
